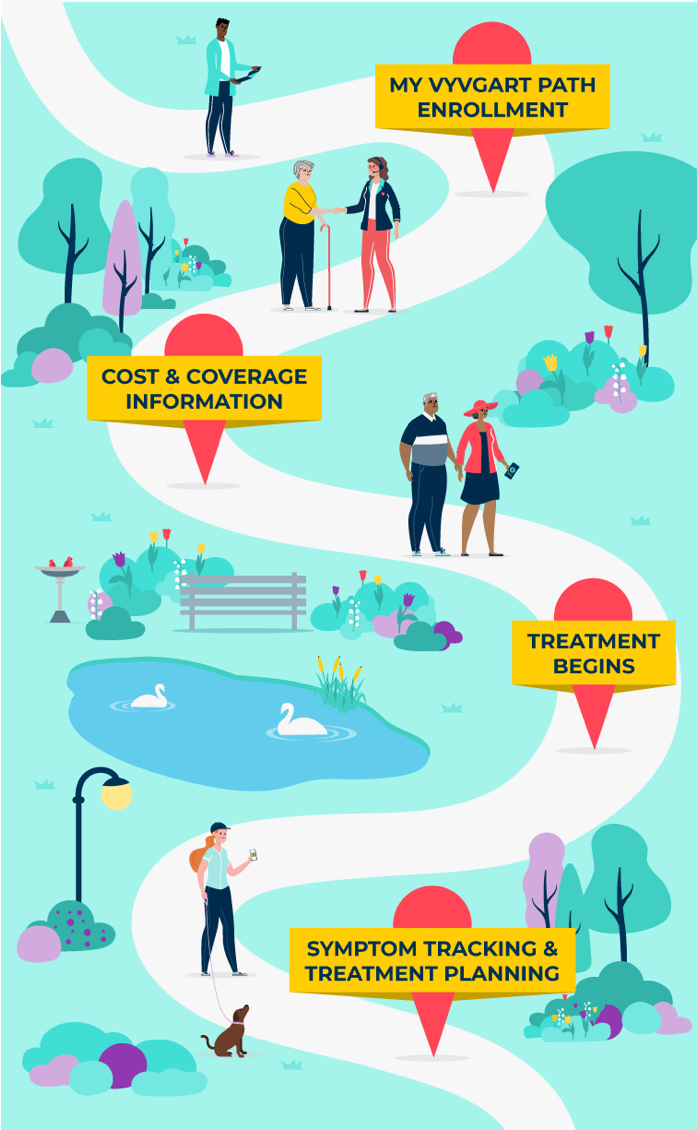
NCM=Nurse Case Manager
Information provided by the Nurse Case Managers is not intended or implied to be a substitute for professional medical advice, diagnosis, or treatment. Always consult with a qualified and licensed physician or other healthcare provider regarding your medical condition, treatment, and healthcare needs.